Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hirata, Sakiko*; Kusaka, Ryoji; Meiji, Shogo*; Tamekuni, Seita*; Okudera, Kosuke*; Hamada, Shoken*; Sakamoto, Chihiro*; Honda, Takumi*; Matsushita, Kosuke*; Muramatsu, Satoru*; et al.
Inorganic Chemistry, 62(1), p.474 - 486, 2023/01
Times Cited Count:0 Percentile:0.01(Chemistry, Inorganic & Nuclear)Haraga, Tomoko; Saito, Shingo*
Bunseki Kagaku, 70(12), p.671 - 679, 2021/12
We developed highly sensitive capillary electrophoresis-laser-induced fluorescence detection methods for lanthanide (Ln) and actinide (An) ions with small sample volume and low emission of waste, by which the radiation risk can be minimized. Specifically, determination of Nd ion in spent nuclear fuel, effective separation between Am and Cm ion, and specific detection of UO
 in real radioactive samples were achieved by molecular design of fluorescence probes composed of an aminocarboxylate chelating moiety, a fluorophore and a spacer, and unique separation mode based on dynamic ternary complexation. We found that there are appropriate combination of probe and ternary complexation for detection and separation of each Ln and An ions. For example, acyclic and macrocyclic hexadentate is suitable for Ln
in real radioactive samples were achieved by molecular design of fluorescence probes composed of an aminocarboxylate chelating moiety, a fluorophore and a spacer, and unique separation mode based on dynamic ternary complexation. We found that there are appropriate combination of probe and ternary complexation for detection and separation of each Ln and An ions. For example, acyclic and macrocyclic hexadentate is suitable for Ln , Am
, Am and Cm
and Cm , and planer tetradentate with
, and planer tetradentate with  electron system is specific for UO
electron system is specific for UO
 , with ppt-sub ppt level detection.
, with ppt-sub ppt level detection.
Kaneko, Masashi; Sasaki, Yuji; Wada, Eriko*; Nakase, Masahiko*; Takeshita, Kenji*
Chemistry Letters, 50(10), p.1765 - 1769, 2021/10
Times Cited Count:0 Percentile:0(Chemistry, Multidisciplinary)Density functional theory calculation is applied to predict the stability constants for Eu and Am
and Am complexes in aqueous solution for molecular modelling of novel separation agents for minor actinides over lanthanides. Logarithm of experimental stability constants correlates with calculated complex formation enthalpies with high reproducibility (R
complexes in aqueous solution for molecular modelling of novel separation agents for minor actinides over lanthanides. Logarithm of experimental stability constants correlates with calculated complex formation enthalpies with high reproducibility (R
 0.98). Prediction of stability constants of novel chelates is demonstrated and indicates a potential availability of the derivatives of diethylenetriaminepentaacetic acid type chelate in acidic condition and enhancement of Am
0.98). Prediction of stability constants of novel chelates is demonstrated and indicates a potential availability of the derivatives of diethylenetriaminepentaacetic acid type chelate in acidic condition and enhancement of Am selectivity over Eu
selectivity over Eu .
.
Sakamoto, Atsushi; Kibe, Satoshi*; Kawanobe, Kazunori*; Fujisaku, Kazuhiko*; Sano, Yuichi; Takeuchi, Masayuki; Suzuki, Hideya*; Tsubata, Yasuhiro; Ban, Yasutoshi; Matsumura, Tatsuro
JAEA-Research 2021-003, 30 Pages, 2021/06
Japan Atomic Energy Agency has been developing a solvent extraction process called SELECT to recover minor actinides (MA) from spent nuclear fuel. In the SELECT process, TDdDGA, HONTA, and ADAAM are used as the extractants for MA + Ln corecovery, MA/Ln separation and Am/Cm separation, respectively. These extractants do not contain phosphorus (P), and consist of carbon (C), hydrogen (H), oxygen (O), and nitrogen (N). In this study, in order to give beneficial information for designing flowsheet, the mass transfer coefficients of Ln between HNO solution and TDdDGA or HONTA / n-dodecane solvent were evaluated by the single drop technique. Prior to the evaluation of mass transfer coefficient, we had optimized the structure of the single drop apparatus to improve accuracy of the measurement. Based on the mass transfer coefficients obtained in HNO
solution and TDdDGA or HONTA / n-dodecane solvent were evaluated by the single drop technique. Prior to the evaluation of mass transfer coefficient, we had optimized the structure of the single drop apparatus to improve accuracy of the measurement. Based on the mass transfer coefficients obtained in HNO / TDdDGA-n-dodecane system, Ln behaviors in the counter-current extraction and back-extraction using mixer-settlers and centrifugal contactors were estimated by simple calculation, and they had a good agreement with our previous experimental results. We also confirmed the mass transfer coefficients of Ln in HNO
/ TDdDGA-n-dodecane system, Ln behaviors in the counter-current extraction and back-extraction using mixer-settlers and centrifugal contactors were estimated by simple calculation, and they had a good agreement with our previous experimental results. We also confirmed the mass transfer coefficients of Ln in HNO / HONTA - n-dodecane system are under 10
/ HONTA - n-dodecane system are under 10 m/s.
m/s.
Kusaka, Ryoji; Watanabe, Masayuki
Journal of Physical Chemistry B, 125(24), p.6727 - 6731, 2021/06
Times Cited Count:8 Percentile:44.55(Chemistry, Physical) /Eu
/Eu selectivity with diethylenetriaminepentaacetic acid and its bisamide chelates.
selectivity with diethylenetriaminepentaacetic acid and its bisamide chelates.Kaneko, Masashi; Sasaki, Yuji; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Journal of Nuclear Science and Technology, 58(5), p.515 - 526, 2021/05
Times Cited Count:3 Percentile:35.51(Nuclear Science & Technology)Density-functional theory calculations were applied to molecular structure and complex formation reaction modelings of metal ion complexes with diethylenetriaminepentaacetic acid (DTPA) and its bisamide (DTPABA) chelates to understand the metal ions selectivity between Am and Eu
and Eu . The calculated complexes with DTPA and DTPABA chelates reproduced the coordination geometries of experimental crystal structures. Calculated Gibbs free energies of the complex formation reactions indicated that Am
. The calculated complexes with DTPA and DTPABA chelates reproduced the coordination geometries of experimental crystal structures. Calculated Gibbs free energies of the complex formation reactions indicated that Am ion forms higher stable complexes with both chelates than Eu
ion forms higher stable complexes with both chelates than Eu ion, being consistent with the experimental results. The higher Am
ion, being consistent with the experimental results. The higher Am selectivity over Eu
selectivity over Eu was suggested to originate in the larger bond overlap between Am
was suggested to originate in the larger bond overlap between Am 5f-orbital and N 2s, 2p-orbital. This mean that the covalent contribution between metal ion and donor atoms differentiates the complex formation stabilities, leading to the Am
5f-orbital and N 2s, 2p-orbital. This mean that the covalent contribution between metal ion and donor atoms differentiates the complex formation stabilities, leading to the Am /Eu
/Eu selectivity. We expect that this study contributes to systematize the origin of metal ions selectivity and to accelerate novel ligands exploration.
selectivity. We expect that this study contributes to systematize the origin of metal ions selectivity and to accelerate novel ligands exploration.
 from nitrate media; Comparison with a conventional organic phosphate
from nitrate media; Comparison with a conventional organic phosphateUeda, Yuki; Kikuchi, Kei*; Tokunaga, Kohei; Sugita, Tsuyoshi; Aoyagi, Noboru; Tanaka, Kazuya; Okamura, Hiroyuki
Solvent Extraction and Ion Exchange, 39(5-6), p.491 - 511, 2021/00
Times Cited Count:0 Percentile:0(Chemistry, Multidisciplinary)no abstracts in English
Kusaka, Ryoji
Journal of Nuclear and Radiochemical Sciences (Internet), 20, p.28 - 31, 2020/06
Kusaka, Ryoji
Hosha Kagaku, (41), p.31 - 33, 2020/03
This commentary article introduced researches involved in encouragement award 2019 of the Japan Society of Nuclear and Radiochemical Sciences. Vibrational sum frequency generation (VSFG) spectroscopy and interfacial studies of solvent extraction of lanthanides and actinides using VSFG spectroscopy were described.
Tsutsui, Nao; Ban, Yasutoshi; Suzuki, Hideya*; Nakase, Masahiko*; Ito, Sayumi*; Inaba, Yusuke*; Matsumura, Tatsuro; Takeshita, Kenji*
Analytical Sciences, 36(2), p.241 - 246, 2020/02
Times Cited Count:21 Percentile:79.8(Chemistry, Analytical)To investigate the effective separation of actinides (Ans) from lanthanides (Lns), single-stage batch extraction experiments were performed with a novel extractant, tetradodecyl-1,10-phenanthroline-2,9-diamide (TDdPTDA) with various diluents such as 3-nitrobenzotrifluoride (F-3), nitrobenzene, and  -dodecane for Am, Cm, and Lns. The extraction kinetics with TDdPTDA was rapid enough to perform the actual extraction flow sheet. The slopes of the distribution ratio versus TDdPTDA concentration and the distribution ratio versus nitric acid concentration were similar for F-3 and nitrobenzene systems but different from
-dodecane for Am, Cm, and Lns. The extraction kinetics with TDdPTDA was rapid enough to perform the actual extraction flow sheet. The slopes of the distribution ratio versus TDdPTDA concentration and the distribution ratio versus nitric acid concentration were similar for F-3 and nitrobenzene systems but different from  -dodecane system. These differences were attributed to the characteristics of the diluents. This study reveals high distribution ratios of Am (
-dodecane system. These differences were attributed to the characteristics of the diluents. This study reveals high distribution ratios of Am (
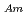 ) and Cm (
) and Cm (
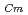 ) for TDdPTDA, with the high separation factors (
) for TDdPTDA, with the high separation factors ( s) of Am from Lns enough for their separation.
s) of Am from Lns enough for their separation.
Sasaki, Yuji; Ban, Yasutoshi; Morita, Keisuke; Matsumiya, Masahiko*; Ono, Ryoma*; Shiroishi, Hidenobu*
Solvent Extraction Research and Development, Japan, 27(1), p.63 - 67, 2020/00
Times Cited Count:6 Percentile:31.74(Chemistry, Multidisciplinary)Mutual separation technique of Dy and Nd in Nd magnet is studied. Dy is more valuable than Nd, then Dy might be isolated and reused. Lanthanide elements can be extracted thoroughly by diglycolamide (DGA) extractants, we use this reagent for the recovery and isolation of Dy. Tetradodecyl-DGA (TDdDGA) has relatively high separation factors(SF) between Dy and Nd (SF=17-18) in HNO extraction system, counter-current extraction using TDdDGA was applied for their mutual separation. From the present study, using the condition, four extraction stages, organic phase: 0.1M TDdDGA in n-dodecane, aqueous phase: 0.3M HNO
extraction system, counter-current extraction using TDdDGA was applied for their mutual separation. From the present study, using the condition, four extraction stages, organic phase: 0.1M TDdDGA in n-dodecane, aqueous phase: 0.3M HNO , 92% Dy can be recovered with 0.7% co-extraction of Nd.
, 92% Dy can be recovered with 0.7% co-extraction of Nd.
 ssbauer isomer shifts using second-order Douglas-Kroll-Hess Hamiltonian
ssbauer isomer shifts using second-order Douglas-Kroll-Hess HamiltonianKaneko, Masashi; Watanabe, Masayuki; Miyashita, Sunao*; Nakashima, Satoru*
Hyperfine Interactions, 239(1), p.20_1 - 20_10, 2018/12
Times Cited Count:4 Percentile:85.02(Physics, Atomic, Molecular & Chemical)We optimized a mixing ratio of exchange energy between pure DFT and exact Hartree-Fock using TPSS exchange-correlation functional to estimate the accurate coordination bonds in f-block complexes by numerically benchmarking with the experimental data of M ssbauer isomer shifts for
ssbauer isomer shifts for 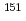 Eu and
Eu and  Np nuclides. Second-order Douglas-Kroll-Hess Hamiltonian with segmented all-electron relativistically contracted basis set was employed to calculate the electron densities at Eu and Np nuclei, i.e. contact densities, for each five complexes for Eu(III) and Np(IV) systems. We compared the root mean square deviation values of their isomer shifts between experiment and calculation by changing the mixing ratio of Hartree-Fock exchange parameter from 0 to 100 % at intervals of 10 %. As the result, it was indicated that the mixing ratio of 30 and 60 % for Eu and Np benchmark systems, respectively, gives the smallest deviation values. Mulliken's spin population analysis indicated that the covalency in the metal-ligand bonds for both Eu and Np complexes decreases with increasing the Hartree-Fock exchange admixture.
Np nuclides. Second-order Douglas-Kroll-Hess Hamiltonian with segmented all-electron relativistically contracted basis set was employed to calculate the electron densities at Eu and Np nuclei, i.e. contact densities, for each five complexes for Eu(III) and Np(IV) systems. We compared the root mean square deviation values of their isomer shifts between experiment and calculation by changing the mixing ratio of Hartree-Fock exchange parameter from 0 to 100 % at intervals of 10 %. As the result, it was indicated that the mixing ratio of 30 and 60 % for Eu and Np benchmark systems, respectively, gives the smallest deviation values. Mulliken's spin population analysis indicated that the covalency in the metal-ligand bonds for both Eu and Np complexes decreases with increasing the Hartree-Fock exchange admixture.
Sasaki, Yuji; Morita, Keisuke
Progress in Nuclear Science and Technology (Internet), 5, p.27 - 32, 2018/12
no abstracts in English
 -Edge X-ray absorption near edge structure spectra
-Edge X-ray absorption near edge structure spectraOta, Atsuyuki*; Tanaka, Kazuya; Tsuno, Hiroshi*
Journal of Physical Chemistry A, 122(41), p.8152 - 8161, 2018/10
Times Cited Count:1 Percentile:3.34(Chemistry, Physical)We investigated the application of L -edge XANES spectra to the local structural analysis of lanthanoids in aqueous solution, iron hydroxide, manganese dioxide, and calcium carbonate. For each lanthanoid, the full width at half maximum (FWHM) values of lanthanoid compounds roughly decreased with increasing coordination numbers. However, they did not strictly reflect the local coordination sphere of the lanthanoid complex, but were rather sensitive to their chemical forms. The relationship between the magnitude of the FWHM values was determined by the crystal field splitting or degeneracy of 5d orbitals. The systematic variation of FWHM can be explained by the ligand strength of the ligand molecules (-H
-edge XANES spectra to the local structural analysis of lanthanoids in aqueous solution, iron hydroxide, manganese dioxide, and calcium carbonate. For each lanthanoid, the full width at half maximum (FWHM) values of lanthanoid compounds roughly decreased with increasing coordination numbers. However, they did not strictly reflect the local coordination sphere of the lanthanoid complex, but were rather sensitive to their chemical forms. The relationship between the magnitude of the FWHM values was determined by the crystal field splitting or degeneracy of 5d orbitals. The systematic variation of FWHM can be explained by the ligand strength of the ligand molecules (-H O
O , -O
, -O , -OH
, -OH , -CO
, -CO
 , -Cl
, -Cl , and -O
, and -O ) that cause the crystal field splitting. Therefore, the FWHM values of L
) that cause the crystal field splitting. Therefore, the FWHM values of L -edge XANES of lanthanoid compounds may be more useful in speciation analysis rather than structural analysis such as EXAFS.
-edge XANES of lanthanoid compounds may be more useful in speciation analysis rather than structural analysis such as EXAFS.
Sato, Tetsuya; Asai, Masato; Borschevsky, A.*; Stora, T.*; Sato, Nozomi*; Kaneya, Yusuke; Tsukada, Kazuaki; D llmann, C. E.*; Eberhardt, K.*; Eliav, E.*; et al.
llmann, C. E.*; Eberhardt, K.*; Eliav, E.*; et al.
EPJ Web of Conferences, 131, p.05001_1 - 05001_6, 2016/12
Times Cited Count:0 Percentile:0.9(Chemistry, Inorganic & Nuclear)Ionization efficiency in a surface ionization process depends on the first ionization potential of the atom. Based on the dependence, the ionization potential of the atom can be determined. We measured ionization efficiencies of fermium, einsteinium, mendelevium, and lawrencium by using a newly developed gas-jet coupled surface ion-source. The ionization potential of the elements have not been determined so far due to their low production rates and/or their short half-lives. Based on a relationship between the ionization efficiency and the ionization potential obtained via measurements of short-lived lanthanide isotopes, the ionization potentials of these actinide elements have been successfully determined.
Sasaki, Yuji; Sugo, Yumi; Morita, Keisuke; Nash, K.*; Nash, K. L.*
Solvent Extraction and Ion Exchange, 33(7), p.625 - 641, 2015/10
Times Cited Count:95 Percentile:91.19(Chemistry, Multidisciplinary)The effect of alkyl substituents at amidic N atoms in diglycolamide (DGA) compounds on solvent extraction was investigated. The solubility in water and n-dodecane, loading capacity, and distribution ratio (D) of lanthanides and actinides for various DGA compounds were monitored. DGA derivatives with short alkyl chains, for example methyl and ethyl groups, are highly water soluble, while DGA derivatives with long alkyl chains are highly soluble in n-dodecane. The loading capacity correlats with their alkyl chain lengths. The results of masking effect and solubility tests indicate that TEDGA(tetraethyl-diglycolamide) is the best actinide masking agent among the water-soluble DGA derivatives. Actinide extractions were performed using ten DGA compounds in six diluents (nitrobenzene, 1,2-dichloroethane, 1-octanol, chloroform, toluene, and n-dodecane), in which it was observed that DGA derivatives with shorter alkyl chains show higher D values.
Ishimori, Kenichiro*; Watanabe, Masayuki; Kimura, Takaumi; Yaita, Tsuyoshi; Yamada, Takashi*; Kataoka, Yumiko*; Shinoda, Satoshi*; Tsukube, Hiroshi*
Chemistry Letters, 34(8), p.1112 - 1113, 2005/08
Times Cited Count:13 Percentile:44.77(Chemistry, Multidisciplinary)Stereochemical substitution on tripod ligand significantly offered efficient separation of trivalent actinides from trivalent lanthanides. Liquid-liquid extraction using chiral tris(2-pyridylmethyl)amine ligands as an extractant exhibited high efficiency and selectivity for trivalent actinides. A combination of chiral ligand and 2-bromodecanoic acid further enhanced extraction performance for trivalent actinides.
Watanabe, Masayuki; Mirvaliev, R.*; Tachimori, Shoichi; Takeshita, Kenji*; Nakano, Yoshio*; Morikawa, Koshi*; Chikazawa, Takahiro*; Mori, Ryohei*
Solvent Extraction and Ion Exchange, 22(3), p.377 - 390, 2004/06
Times Cited Count:32 Percentile:65.75(Chemistry, Multidisciplinary)The synergistic extraction with N,N,N',N'-tetrakis(2-methylpyridyl)-ethylenediamine (TPEN) and di(2-ethylhexyl)phosphoric acid (D2EHPA) demonstrates a good selectivity for Am(III) over macro amount of Ln(III) in 1-octanol. The maximum apparent separation factor, which is defined as the ratio of the distribution ratio of Am(III) to that of Eu(III), is ca. 80 while the molar fraction between D2EHPA and TPEN is 2.0. This ratio is corresponding to the result of the spectrophotometric titration, which indicates that TPEN and D2EHPA form an aggregate in 1-octanol and two D2EHPA molecules coordinate to Eu(III) TPEN complex, [Eu(TPEN)] . In the present study, the association of D2EHPA and TPEN is one of the most important factors for the synergistic extraction and the complexation of TPEN and D2EHPA with Eu(III).
. In the present study, the association of D2EHPA and TPEN is one of the most important factors for the synergistic extraction and the complexation of TPEN and D2EHPA with Eu(III).
Dobler, M.; Hirata, Masaru
Physical Chemistry Chemical Physics, 6(8), p.1672 - 1678, 2004/04
Times Cited Count:4 Percentile:11.98(Chemistry, Physical)no abstracts in English
Zhang, P.*; Kimura, Takaumi; Yoshida, Zenko
Solvent Extraction and Ion Exchange, 22(6), p.933 - 945, 2004/00
Times Cited Count:13 Percentile:45.29(Chemistry, Multidisciplinary)no abstracts in English